Supramolecular recognition and sensing of nucleotides, RNA, and DNA by synthetic receptors
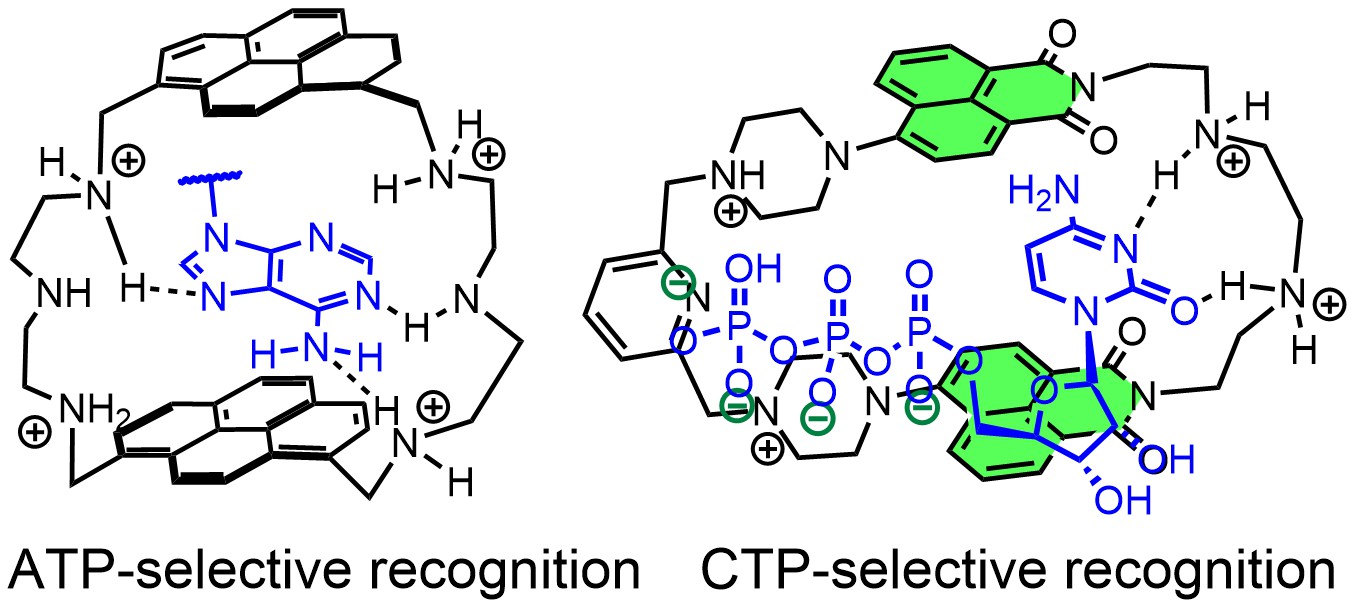
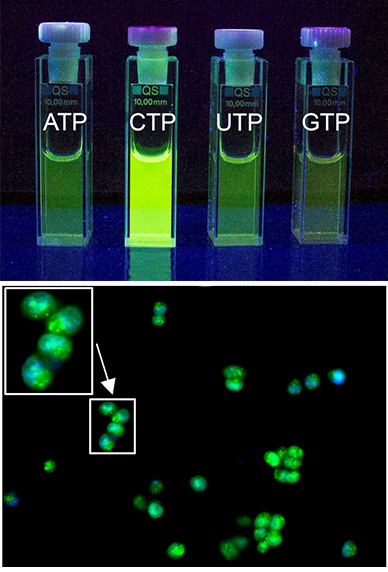 In this project, we design synthetic receptors targeting single nucleotides and longer sequences. In most systems, we incorporate fluorescent dyes to detect host-guest complexes in biological medium. We mainly explore non-covalent interactions between nucleobases and designed artificial receptors to understand the binding strength, geometry of complexes, and selectivity of the generated fluorometric response. Fluorescent receptors for recognizing unusual secondary nucleic acid structures are also our focus. The ultimate goal is to achieve high-affinity receptors to control replication and DNA-protein interactions (Angew. Chem. Int. Ed. 2023). For instance, we developed a new bellows-type sensing mechanism for nucleotide detection in an aqueous solution based on the pyrene-containing cyclophane for ratiometric detection of ATP (Chem. Eur. J. 2020, JACS 2024). Another series of cyclophanes can bind to RNA sequences, specifically those containing C-rich subunits. Our naphthalimide-containing cyclophanes can selectively encapsulate free cytosine and cytidine phosphates in an aqueous solution with up to 60-fold fluorescence enhancement (JACS au 2023). These receptors are effective against breast cancer cells.
In this project, we design synthetic receptors targeting single nucleotides and longer sequences. In most systems, we incorporate fluorescent dyes to detect host-guest complexes in biological medium. We mainly explore non-covalent interactions between nucleobases and designed artificial receptors to understand the binding strength, geometry of complexes, and selectivity of the generated fluorometric response. Fluorescent receptors for recognizing unusual secondary nucleic acid structures are also our focus. The ultimate goal is to achieve high-affinity receptors to control replication and DNA-protein interactions (Angew. Chem. Int. Ed. 2023). For instance, we developed a new bellows-type sensing mechanism for nucleotide detection in an aqueous solution based on the pyrene-containing cyclophane for ratiometric detection of ATP (Chem. Eur. J. 2020, JACS 2024). Another series of cyclophanes can bind to RNA sequences, specifically those containing C-rich subunits. Our naphthalimide-containing cyclophanes can selectively encapsulate free cytosine and cytidine phosphates in an aqueous solution with up to 60-fold fluorescence enhancement (JACS au 2023). These receptors are effective against breast cancer cells.
Functional fluorescent dyes and dynamic combinatorial chemistry
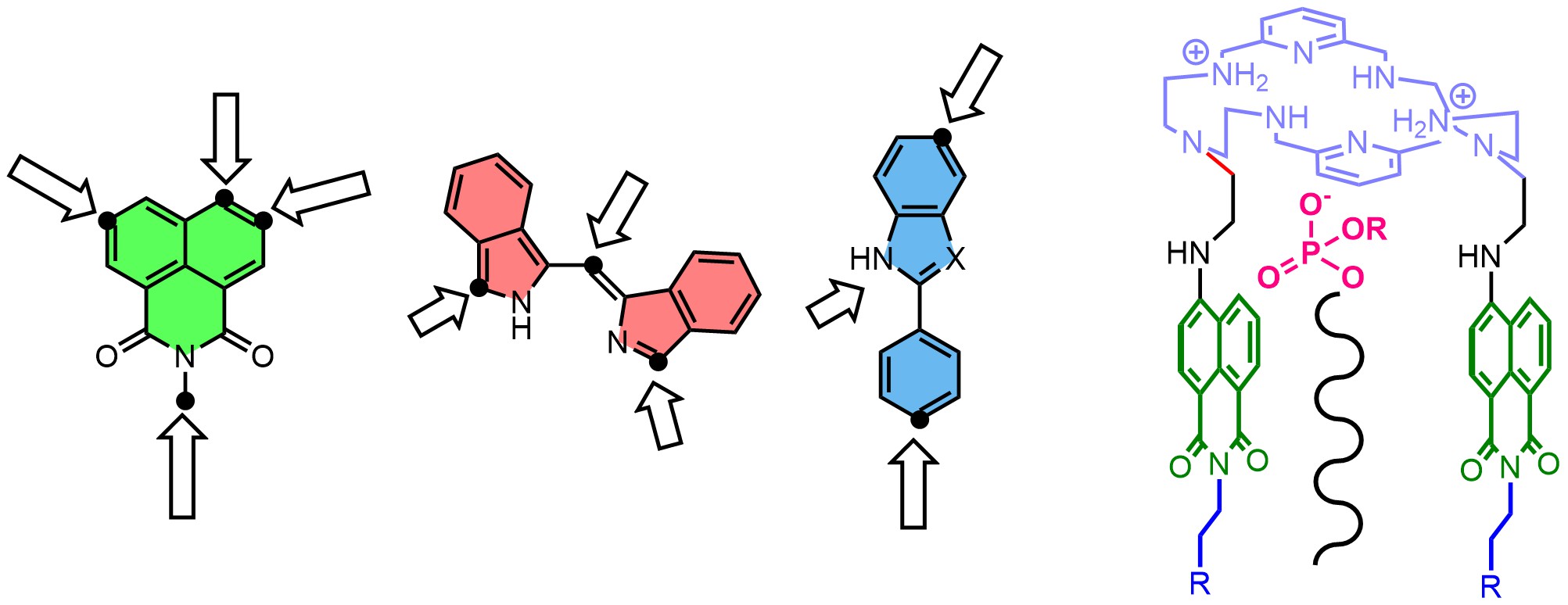 In many projects, we synthesize and functionalize fluorescent dyes to conjugate them with supramolecular recognition subunits. These functionalized dyes are used to detect and visualize biologically important anions (Chem. Eur. J. 2018), cations (Sensor. Actuat. B-Chem, 2020), gases (Chem. Comm. 2022), and biomembranes (Sensor. Actuat. B-Chem. 2023).
In many projects, we synthesize and functionalize fluorescent dyes to conjugate them with supramolecular recognition subunits. These functionalized dyes are used to detect and visualize biologically important anions (Chem. Eur. J. 2018), cations (Sensor. Actuat. B-Chem, 2020), gases (Chem. Comm. 2022), and biomembranes (Sensor. Actuat. B-Chem. 2023).
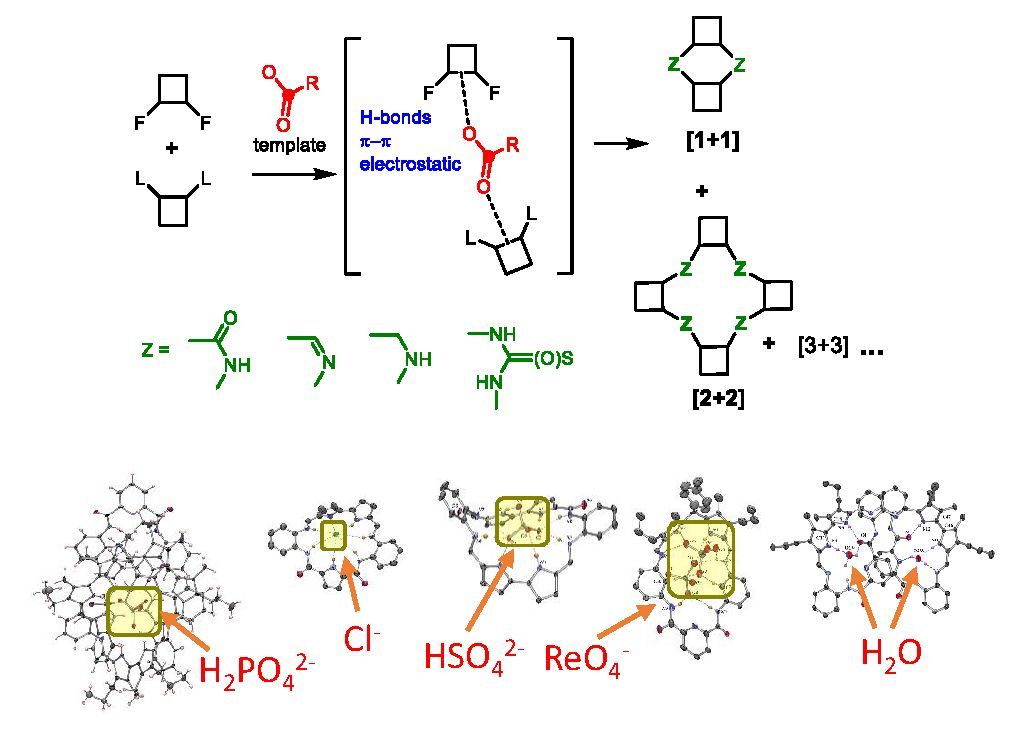 The new supramolecular receptors are created using dynamic combinatorial chemistry and template synthesis. We utilize both reversible and irreversible covalent bonds to link subunits that contain hydrogen bond donors or acceptor groups with fluorescent dyes. This process allows us to develop a fluorescent receptor for a specific target species. The receptor can function as a supramolecular or artificial transporter, probe, catalyst, or messenger. Our strategy for the design of anion-selective fluorescent probes is based on the supramolecular pKa shift of amino-groups placed in proximity to dyes (Chem. Comm. 2023). We developed probes for many anions, such as chloride, fluoride, phosphate, oxalate, and pyrophosphate.
The new supramolecular receptors are created using dynamic combinatorial chemistry and template synthesis. We utilize both reversible and irreversible covalent bonds to link subunits that contain hydrogen bond donors or acceptor groups with fluorescent dyes. This process allows us to develop a fluorescent receptor for a specific target species. The receptor can function as a supramolecular or artificial transporter, probe, catalyst, or messenger. Our strategy for the design of anion-selective fluorescent probes is based on the supramolecular pKa shift of amino-groups placed in proximity to dyes (Chem. Comm. 2023). We developed probes for many anions, such as chloride, fluoride, phosphate, oxalate, and pyrophosphate.
Molecular switches and artificial signal transduction
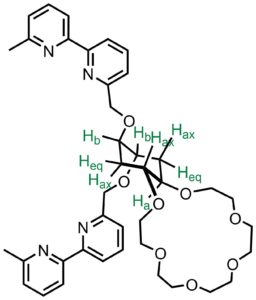 Molecular switches are important topics for signal-processing at molecular level. We are looking at the molecular transformations in an unconventional way by combining molecular recognition events in one assembly to achieve such properties as reading, writing, memory, switching, logic operations and etc. We have developed a first cation molecular exchanger based on the cyclohexane ring. We have demostrated that binding of the zinc(II) cation to the bipyridine subunit induces the conformational switching of the crown ether subunits, which subsequently results in a release of the potassium cation. The release of the potassium cation can be detected by a cation sensor. (Org. Lett. 2018, 20 (19), 6211–6214)
Molecular switches are important topics for signal-processing at molecular level. We are looking at the molecular transformations in an unconventional way by combining molecular recognition events in one assembly to achieve such properties as reading, writing, memory, switching, logic operations and etc. We have developed a first cation molecular exchanger based on the cyclohexane ring. We have demostrated that binding of the zinc(II) cation to the bipyridine subunit induces the conformational switching of the crown ether subunits, which subsequently results in a release of the potassium cation. The release of the potassium cation can be detected by a cation sensor. (Org. Lett. 2018, 20 (19), 6211–6214)
Synthesis and properties of curved aromatics
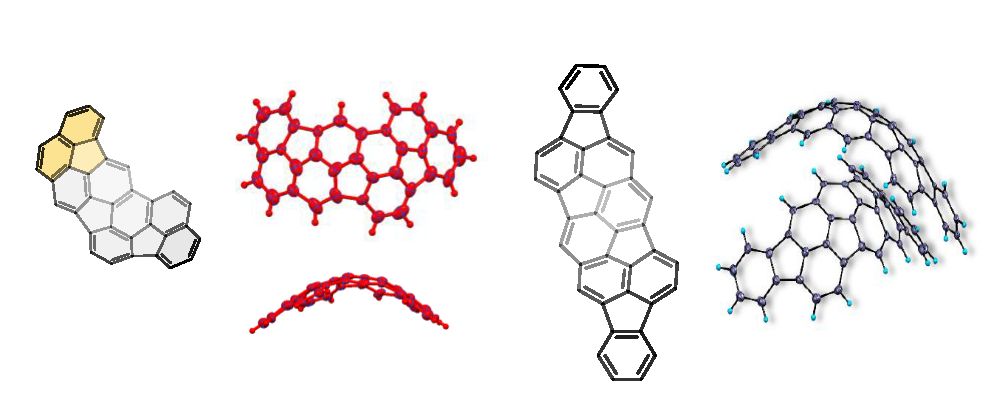 This project aims to develop a new family of bowl-shape and macrocyclic PAHs, which can bind diverse guest molecules, involving those of biological relevance, such as amino acids and protein surfaces, and function as supramolecular catalysts. We have recently designed a series of new curved PAHs and supramolecular hosts in collaboration with project partners. The latter bear elementary binding sites (hydrogen bond donors, acceptors, or charged groups).
This project aims to develop a new family of bowl-shape and macrocyclic PAHs, which can bind diverse guest molecules, involving those of biological relevance, such as amino acids and protein surfaces, and function as supramolecular catalysts. We have recently designed a series of new curved PAHs and supramolecular hosts in collaboration with project partners. The latter bear elementary binding sites (hydrogen bond donors, acceptors, or charged groups).
